

On the basis of the above concept it is, however, not easy to explain the irregularities found in the observed electronic configurations of the atoms of other elements, since one has to consider the net effect of so many other factors such as

The irregularities in the observed configurations of Cr (3d 54s 1), Cu (3d 104s 1), Mo (4d 55s 1), Pd (4d 105s 0), Ag (4d 10 5s 1) and Au (5d 106s 1) are explained on the basis of the concept that half-filled and completely filled d-orbitals are relatively more stable than other d-orbitals.
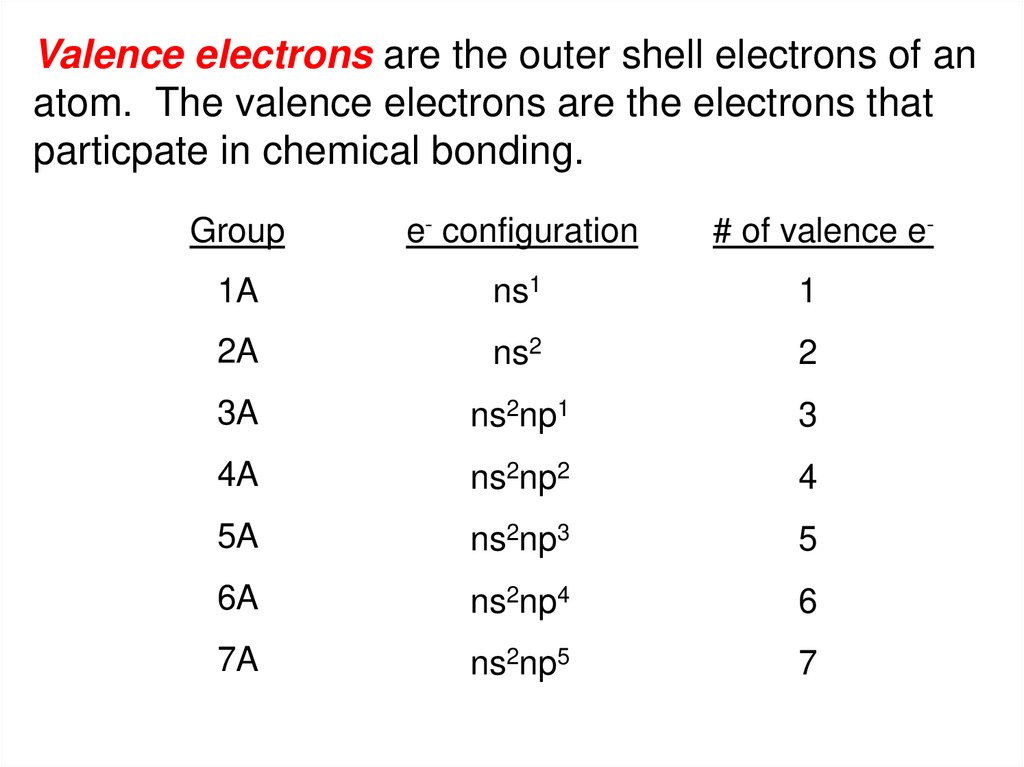
ATOM WITH NS2 ELECTRON CONFIGURATION SERIES
Each of 3d, 4d and 5d series has ten elements while 6d series has at present only one element viz Ac80 whose valence shell configuration is 6d 1 7s 2. Yet, in order to maintain a rational classification of elements, these elements (viz Cu, Ag, Au, Zn, Cd, Hg and Pd) are also generally studied with d-block elements.Īll the d-block elements are classified into four series viz 3d, 4d, 5d and 6d series corresponding to the filling of 3d, 4d, 5d and 6d orbitals of (n-1)th main shell. Similar is the case with Pd atom with configuration 4d 105s0. Similarly Zn, Cd and Hg which both in their atomic state and in +2 oxidation do not contain partly filled (n-1)d orbitals, should also be excluded from d-block elements. The configurations clearly show that strictly, according to the definition of d-block elements, Cu, Ag and Au should be excluded from d-block elements, since these elements, both in their atomic state and in their +1 oxidation state, do not have partly filled (n – 1)d-orbitals. The valence shell configurations of these elements can be represented by (n-1)d 1-10.ns0, 1, 2. In these elements the differentiating electron enters (n-1)d orbitals of (n-1)th main shell and as such these are called d-block elements. These elements either in their atomic state or in any of their common oxidation state have partly filled (n-1) d-orbitals of (n-1)th main shell. Definition and Electronic Configurations of Atoms The elements lying between s and p-block elements of the periodic table are collectively known as transition or transitional elements (T.E.’s):


 0 kommentar(er)
0 kommentar(er)
